Introduction
In the rapidly evolving landscape of cancer therapy, Antibody-Drug Conjugates (ADCs) have emerged as groundbreaking precision medicines that marry the targeting ability of antibodies with the potent cell-killing power of cytotoxic drugs. This innovative approach has revolutionized oncologic treatment paradigms by maximizing anti-tumor efficacy while minimizing systemic toxicity. As researchers and clinicians continue to refine ADC technologies, these targeted therapies offer new hope for patients facing difficult-to-treat cancers.
Definition
Antibody–Drug Conjugate (ADC) Oncology refers to a class of targeted cancer therapies that combine a monoclonal antibody specific to a tumor-associated antigen with a potent cytotoxic drug linked via a chemical linker. The antibody selectively delivers the drug to cancer cells, allowing internalization and controlled release of the cytotoxic payload, thereby maximizing tumor cell killing while minimizing damage to healthy tissues.
What Are Antibody-Drug Conjugates?
Antibody-Drug Conjugates (ADCs) are an advanced class of biopharmaceutical agents designed to selectively deliver cytotoxic drugs directly into cancer cells. Structurally, an ADC consists of three critical components:
- Monoclonal Antibody (mAb) – This is the targeting arm of the ADC. It specifically recognizes and binds to an antigen uniquely or highly expressed on the surface of cancer cells.
- Cytotoxic Payload (Warhead) – These are powerful chemotherapy agents designed to kill cancer cells. When delivered in free form, these agents are often too toxic for systemic administration.
- Linker – A specialized chemical bridge that connects the antibody to the cytotoxic payload. Linker design is crucial: it must be stable enough to prevent premature drug release in the bloodstream, yet cleavable once inside the tumor cell.
The concept behind ADCs is elegant – harness the specificity of antibodies to ferry highly potent drugs straight to the tumor, dramatically enhancing therapeutic index and reducing damage to healthy tissues.
How Do ADCs Work?
The mechanism of ADCs follows a precise, multi-step process:
- Target Recognition and Binding: Once administered intravenously, the monoclonal antibody portion of the ADC selectively binds to its target antigen on the cancer cell surface.
- Internalization: The antibody–antigen complex is then internalized into the cancer cell through endocytosis.
- Linker Cleavage and Drug Release: Once inside, the linker is enzymatically or chemically cleaved, liberating the cytotoxic drug.
- Tumor Cell Kill: The released payload exerts its cell-killing effect, typically by interfering with DNA replication or microtubule function, ultimately leading to cancer cell death.
This sequence ensures that potent chemotherapy drugs act where they are needed most – inside cancer cells – dramatically reducing off-target toxicity.
Why ADCs Are Considered Precision Medicine
Precision medicine aims to tailor treatment to individual patients based on genetic, biomarker, phenotypic, and environmental factors. ADCs embody this principle in oncology:
- They are biomarker-driven – designed only for cancers that express the specific target antigen.
- They allow selective delivery of treatment, sparing normal cells.
- They represent an evolution beyond traditional chemotherapy, offering more effective and less toxic therapeutic options.
This precision approach improves patient outcomes while enhancing quality of life – a critical consideration in cancer therapy.
Clinical Successes of ADCs
Several ADCs have already gained regulatory approval and transformed treatment options across diverse cancer types:
- Brentuximab vedotin — Approved for Hodgkin lymphoma and systemic anaplastic large cell lymphoma, this ADC targets CD30 and has shown significant clinical benefit. It delivers the potent antimitotic agent MMAE (monomethyl auristatin E), improving outcomes in both frontline and relapsed settings.
- Trastuzumab emtansine (T-DM1) — Designed for HER2-positive breast cancer, this ADC combines trastuzumab with the cytotoxic DM1. It has become a cornerstone in treating metastatic HER2-positive disease by improving survival with reduced toxicity compared to conventional chemotherapy.
- Enfortumab vedotin — A CD-weighted ADC for advanced urothelial carcinoma, providing new therapeutic options for patients with limited alternatives.
- Polatuzumab vedotin and Sacituzumab govitecan — Targeting CD79b and Trop-2 respectively, these ADCs have expanded treatment approaches in diffuse large B-cell lymphoma and metastatic triple-negative breast cancer.
These successes highlight how ADCs are shifting clinical practice, offering targeted therapies where traditional treatments fall short.
Challenges and Limitations
While ADCs represent a powerful therapeutic platform, they are not without challenges:
Antigen Selection:
Identifying a target that is abundant on tumor cells but absent or low on normal tissues is critical. Poor antigen selection can lead to off-target effects and reduced therapeutic windows.
Linker Stability:
Better linkers mean controlled drug release only inside cancer cells. Premature release in circulation can cause systemic toxicity, while overly stable linkers may hinder drug activation.
Resistance Mechanisms:
Cancer cells, like with many therapies, can develop resistance through:
- Antigen downregulation
- Drug efflux pumps
- Altered intracellular processing
Overcoming resistance remains an active area of research.
Toxicity Considerations:
Despite their targeted design, ADCs can still cause side effects, such as:
- Neutropenia
- Peripheral neuropathy
- Hepatotoxicity
Refinements in design and dosing strategies are continually aimed at reducing these toxicities.
Emerging Innovations in ADC Technology
Advances in ADC research aim to improve efficacy, safety, and therapeutic reach:
Next-Generation Payloads:
New cytotoxic agents with different mechanisms of action are being explored, including:
- DNA alkylators
- Topoisomerase inhibitors
These expand the arsenal of warheads beyond traditional microtubule disruptors.
Enhanced Linkers:
Cleavable linkers triggered by conditions like low pH, specific enzymes, or redox status improve selective drug release with minimal systemic leakage.
Bispecific and Multivalent ADCs:
These ADCs can recognize multiple tumor antigens, enhancing selectivity and overcoming heterogeneity within tumors.
Combination Therapies:
ADCs are being paired with immunotherapies (like checkpoint inhibitors) and targeted small molecules to potentiate anti-tumor effects.
Patient Impact: More Than Just Numbers
Beyond clinical trial statistics, the impact of ADC therapy on patients’ lives cannot be overstated. Many patients experience:
- Improved survival outcomes
- Reduced side effects compared with traditional chemotherapy
- Enhanced quality of life
- New hope for refractory or aggressive cancers
For patients with limited options, ADCs are not just treatments – they represent new chapters in the fight against cancer.
Future Trends of Antibody-Drug Conjugate (ADC) Oncology Market
Expansion into Solid Tumors:
The ADC oncology market is expected to witness strong growth as next-generation ADCs expand beyond hematological malignancies into a wider range of solid tumors, including lung, gastric, ovarian, and colorectal cancers, driven by improved target selection and enhanced tumor penetration.
Advancements in ADC Technology:
Innovations in linker chemistry, novel cytotoxic payloads, and site-specific conjugation techniques are enhancing ADC stability, efficacy, and safety, enabling the development of more potent and better-tolerated therapies.
Rising Personalized Medicine Adoption:
As precision oncology advances, ADC development is increasingly aligned with biomarker-driven patient selection, ensuring higher treatment success rates and reinforcing the role of ADCs in personalized cancer care.
Growth in Combination Therapies:
Future market trends indicate increased use of ADCs in combination with immunotherapies, targeted agents, and chemotherapy, aiming to overcome resistance mechanisms and improve long-term clinical outcomes.
Growth Rate of Antibody-Drug Conjugate (ADC) Oncology Market
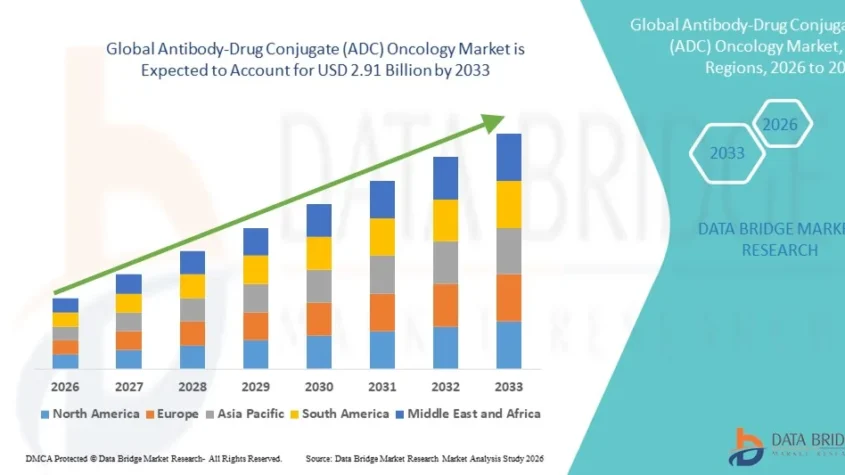
According to Data Bridge Market Research, the Antibody-Drug Conjugate (ADC) oncology market was estimated to be worth USD 1.28 billion in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 10.80% to reach USD 2.91 billion by 2033.
Read More: https://www.databridgemarketresearch.com/reports/global-antibody-drug-conjugate-adc-oncology-market
Conclusion
Antibody-Drug Conjugates have transformed the oncology landscape by delivering targeted, potent therapies with improved safety profiles. From their biological sophistication to their real-world impact, ADCs represent a hallmark of precision medicine. As science continues to innovate, ADCs will undoubtedly play an increasingly central role in cancer treatment — offering hope, improving outcomes, and redefining therapeutic possibilities.
Discover Modern Comfort: A Guide to Apartment Rent in Beirut
Beirut, the lively capital of Lebanon, is a city that blends deep-rooted history with a mo…